Differential cartilage turnover along the human lower limb revealed by protein deamidation
M.-F. Hsueh Osteoarthritis and Cartilage VOLUME 26, SUPPLEMENT 1, S32, APRIL 01, 2018
Purpose: It is believed that humans do not have sufficient intrinsic repair responses for dealing with cartilage injuries. However, enriched progenitor cells have been reported in the superficial layer of osteoarthritis (OA) cartilage, although the extent to which these cells participate in tissue repair is unclear. We hypothesize that quantification of post-translational modifications of proteins could provide a means of determining the turnover of proteins in vivo. We focused on deamidation, a non-enzymatic covalent amino acid modification caused by the hydrolysis of the amide group of Asn or Gln to form Asp and Glu. In the present study, we utilized proteomic tools to profile (to identify and quantify) deamidated epitopes within cartilage extracellular matrix (ECM) proteins and to quantify their variation by different biological factors including different joint sites, disease states, and cartilage depths.
Methods: Under Duke Institutional Review Board approval, full-thickness cartilage from ankle, knee, and hip joints (both healthy and OA with matched age range; n = 3 replicates) were obtained. Serial transverse cryosections were generated at different depths from the surface of cartilage. Cartilage ECM proteins were extracted by guanidine-HCl and then prepared for mass spectrometry analysis, which was performed with a HPLC-connected QExactive mass spectrometer. We calculated the standardized deamidation ratio (deamidated Asn/Total Asn), and took the mean standardized values of the peptides derived from a protein to represent the overall abundance of the deamidated form of a protein. Multivariable analyses were performed to evaluate for differences in abundance of deamidated epitopes due to all biological factors. We estimated the protein half-life based on an algorithm derived from prior published studies and the deamidation data.
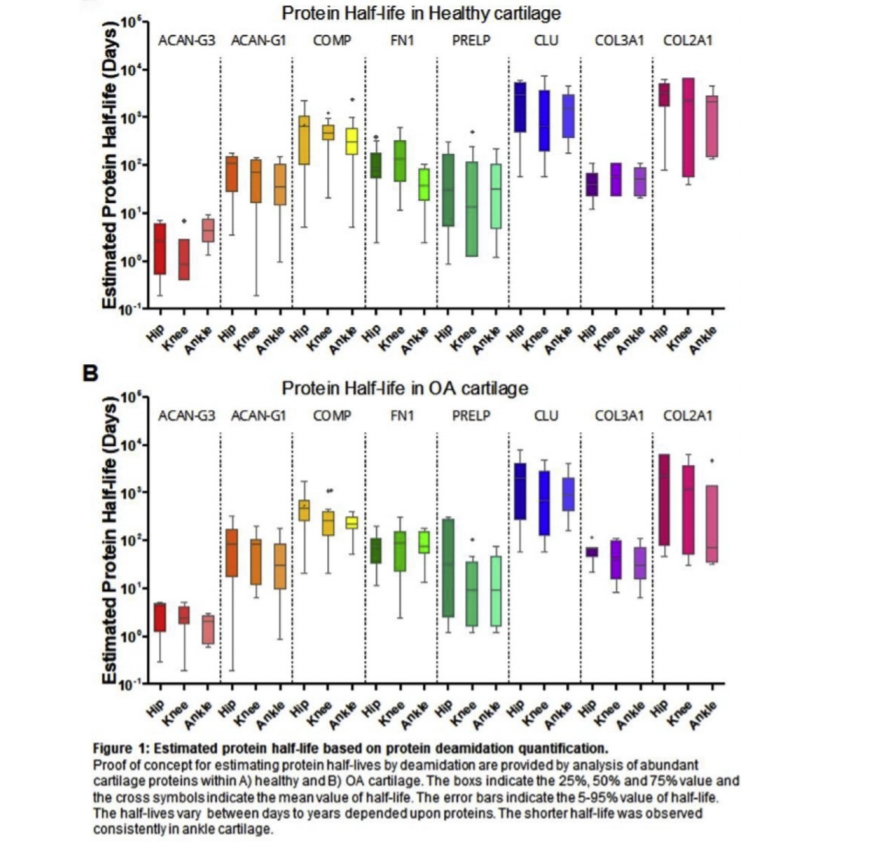
Results: Out of a total of 469 proteins identified in cartilage from all three joints, 285 proteins (61%) contained at least one deamidated residue. Compared to the mean standardized (Z-score) value, cartilage of hip joints had the most deamidated residues followed by knee then ankle joint cartilage (Table 1). Deamidated proteins were most abundant in the deep layer of cartilage (Table 1). The abundance of deamidated proteins was lower in OA cartilage than healthy cartilage for all monitored proteins. Based on these results and prior published methodology, we estimated the protein half-life and found that protein half-lives ranged from days to years depending upon the protein identity (Fig. 1). Within the healthy cartilage, the shortest half-life, 3.4 days (95% confidence interval [CI: 2.0-4.7]), was observed for the aggrecan G3 domain. Interestingly, the amino terminus of aggrecan (G1 domain), known to be retained in the cartilage matrix after metalloproteinase and ADAMTS proteolysis of aggrecan, had a longer half-life, 78.9 days (CI: 60.2–97.6). The pool of collagen type II (that we were able to extract from articular cartilage by GuHCl) had the longest half-life of the seven ECM proteins evaluated in this study: healthy cartilage 2779 days, CI: 1618–3941; OA cartilage 1930 days, CI: 700–3161. Beside protein identity, the next major determinant of protein half-lives was joint site (ankle<knee<hip).
Conclusions: Deamidated proteins are abundant in cartilage and enriched in the ECM. We established an integrated algorithm to estimate the turnover rate of different proteins based on deamidation. As a proof of concept, the half-lives of seven articular cartilage proteins were estimated. Among these proteins, collagen type II had the slowest turnover rate and the aggrecan carboxy-terminus the highest. Cartilage protein turnover varied by joint site (highest in ankle), disease state (highest in OA), and cartilage depth (highest in the superficial layer). A general reduction of deamidated epitopes in ankle/knee cartilage and the superficial layer of cartilage likely indicates a hitherto unappreciated intrinsic regenerative tissue gradient response along the lower limb and an endogenous repair response of cartilage that is upregulated in OA whose mechanisms warrant further exploration.