A double-blind, placebo-controlled, randomised, clinical study on the effectiveness of collagen peptide on osteoarthritis
Suresh Kumar Journal of the Science of Food and Agriculture 2014 Early View
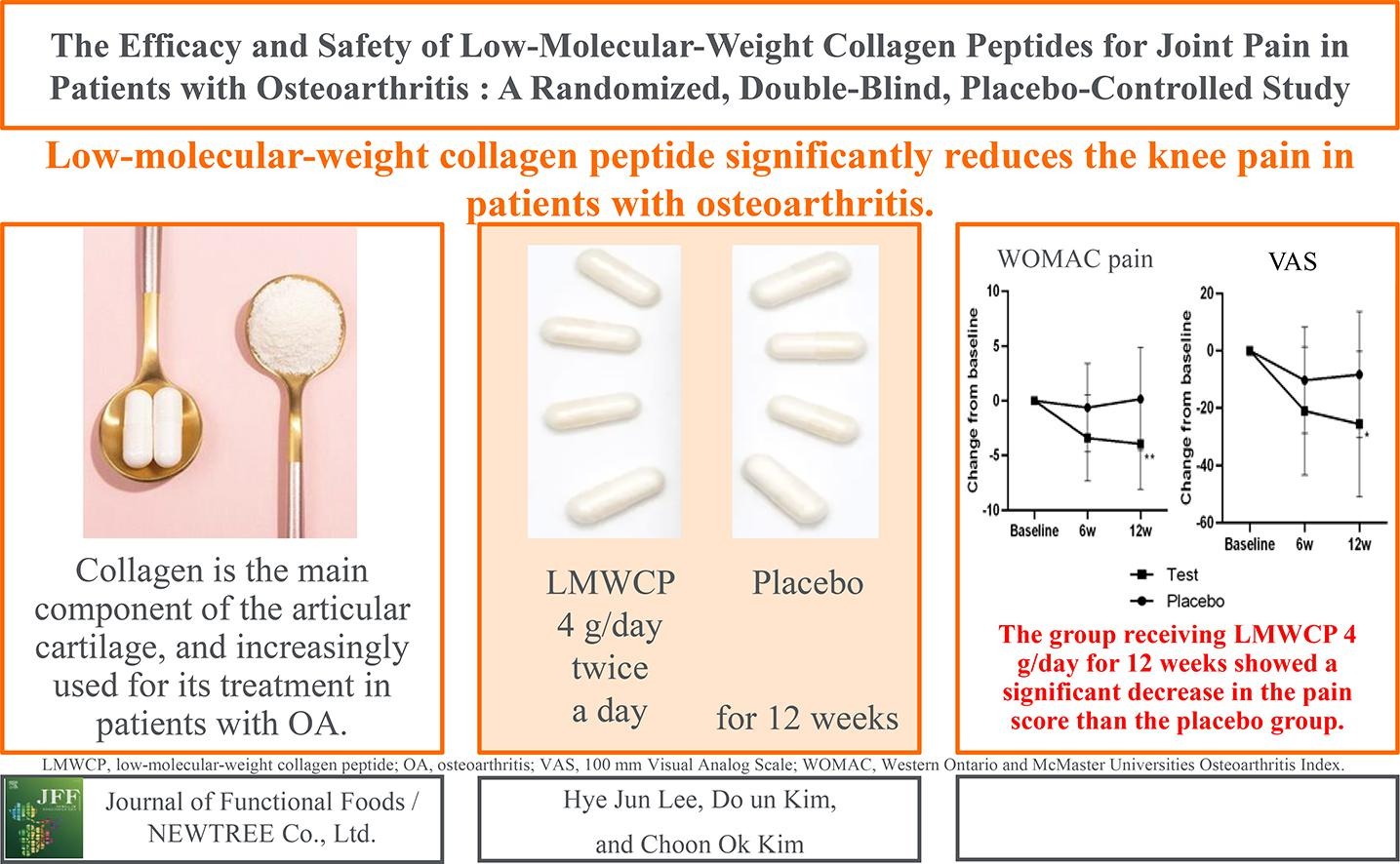
Recent studies show that enzymatically hydrolysed collagen, the collagen peptide, is absorbed and distributed to joint tissues and has analgesic and anti-inflammatory properties. A double-blind, placebo-controlled, randomised trial with collagen peptides isolated from pork skin (PCP) and bovine bone (BCP) sources was carried out to study the effectiveness of orally supplemented collagen peptide to control the progression of osteoarthritis in patients diagnosed with knee osteoarthritis. Improvement in treatment was assessed with reduction in Western Ontario McMaster Universities (WOMAC), visual analogue scale (VAS) and quality of life (QOL) scores from baseline to 13 weeks (Visit 7). Safety and tolerability were also evaluated.
RESULTS
There was significant reduction from baseline to Visit 7 in the primary end points of WOMAC and VAS scores and in the secondary end point of QOL score in subjects with PCP and BCP groups, while in subjects with placebo group the end point indices remained unaltered. Furthermore, all the score levels of WOMAC, VAS and QOL decreased significantly (P < 0.01) in the study group compared to placebo group in Visit 7.
CONCLUSION
The study demonstrated that collagen peptides are potential therapeutic agents as nutritional supplements for the management of osteoarthritis and maintenance of joint health.