Carnitine Acetyltransferase Mitigates Metabolic Inertia and Muscle Fatigue during Exercise
Sarah E. Seiler Cell Metab Volume 22, Issue 1, p65–76, 7 July 2015
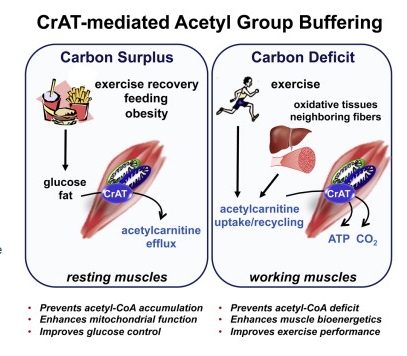
Highlights
•CrAT is a mitochondrial enzyme that interconverts acetyl-CoA and acetylcarnitine
•Acetylcarnitine in blood and tissues has emerged as a biomarker of energy surplus
•Muscle contraction stimulates acetylcarnitine uptake, recycling, and oxidation
•CrAT-mediated acetyl group buffering is essential for optimal exercise performance
Summary
Acylcarnitine metabolites have gained attention as biomarkers of nutrient stress, but their physiological relevance and metabolic purpose remain poorly understood. Short-chain carnitine conjugates, including acetylcarnitine, derive from their corresponding acyl-CoA precursors via the action of carnitine acetyltransferase (CrAT), a bidirectional mitochondrial matrix enzyme.
We show here that contractile activity reverses acetylcarnitine flux in muscle, from net production and efflux at rest to net uptake and consumption during exercise. Disruption of this switch in mice with muscle-specific CrAT deficiency resulted in acetyl-CoA deficit, perturbed energy charge, and diminished exercise tolerance, whereas acetylcarnitine supplementation produced opposite outcomes in a CrAT-dependent manner. Likewise, in exercise-trained compared to untrained humans, post-exercise phosphocreatine recovery rates were positively associated with CrAT activity and coincided with dramatic shifts in muscle acetylcarnitine dynamics. These findings show acetylcarnitine serves as a critical acetyl buffer for working muscles and provide insight into potential therapeutic strategies for combatting exercise intolerance.













