Vascular effects of insulin
Andrea Natali metabol. 2021.154891
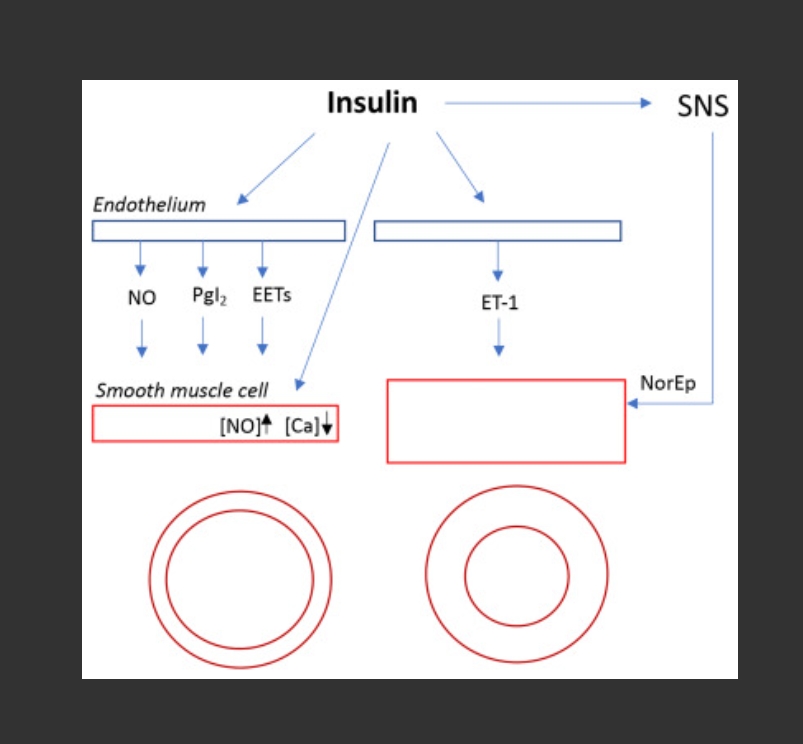
Insulin affects the vascular tone through multiple and complex mechanisms involving both vasodilator and vasoconstrictor pathways (Fig. 1) that are further regulated by specific physiologic and pathologic conditions, including insulin resistance. Insulin receptors are present on both endothelial and smooth muscle cells. In the former, binding to the receptor leads to the activation of nitric oxide (NO) synthase and the release of prostaglandin I2 (PgI2) through the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signaling, while through the RAF/mitogen activated protein kinase (MAPK) signaling cascade the synthesis of Endothelin-1 is enhanced. An activation of arachidonic acid metabolites with hyperpolarizing action, the epoxeicosotrianoic acids (EETs), has also been reported in both experimental animals [ [1] ] and humans [ [2] ].
These actions in vitro are particularly evident when cells are exposed to pharmacologic insulin concentrations [ [3] ] whose molecular aspects are reviewed in detail by recent papers [ 4 , 5 , 6 ]. In vivo, at least in resting human skeletal muscle tissue, the three major systems (NO, PgI2, and Endothelin-1) are tonically activated, being responsible for the control of approximately 20–30% of basal blood flow (BF) [ 7 , 8 , 9 ], and are modulated by a series of physiologic stimuli (exercise, ischemia, inflammation, free fatty acids, hyperglycemia), hormonal factors (insulin growth factor-1 [IGF1], vasopressin, adrenergic, and cholinergic mediators, bradykinin), as well as by pharmacologic agents. Noteworthy, insulin is also able to stimulate NO synthesis in smooth muscle cells [ [10] ], to attenuate the alpha-adrenergic vasoconstriction, while potentiating β-adrenergic dilatation [ [11] ]. Finally, in conditions of systemic euglycemic hyperinsulinemia there is an increase in peripheral sympathetic discharge [ [12] ] and in norepinephrine spillover [ [11] ], which increase the vascular tone through the α-adrenoceptors.