Are all sugar pills created equal? A systematic review and network meta-analysis
R.R. Bannuru Osteoarthritis and Cartilage VOLUME 26, SUPPLEMENT 1, S27, APRIL 01, 2018
Purpose: Placebo-controlled randomized controlled trials (RCTs) are the highest level of evidence with which to assess the relative benefits of any treatment. However, the placebo effects that are inevitably observed in these trials must be taken into account when interpreting the results, because an overall treatment’s effect size is derived from the difference between the effect seen in the treatment group and the placebo effect. Sugar pills are used as placebo interventions in trials examining both oral pharmaceutical and non-pharmaceutical (i.e. nutraceutical or herbal) treatments for knee OA. However, systematic variation in the magnitude of response among these two types of sugar pills would have important implications for the interpretation of placebo-controlled trials, and our knowledge base on this issue is limited. Our systematic review and network meta-analysis of RCTs in knee OA aimed to ascertain the potential for differential placebo effects observed in RCTs of oral pharmaceutical vs. non-pharmaceutical treatments.
Methods: We searched Medline, EMBASE, Web of Science, Google Scholar, and Cochrane Database from inception to November 2017 for RCTs of adults with knee OA that compared oral pharmaceutical or non-pharmaceutical treatments against each other and/or against a matching placebo tablet or capsule. Unpublished data were actively sought. Data were extracted independently by two reviewers who assessed study quality with the Cochrane risk of bias tool. We calculated standardized mean differences (SMD) for pain at 2, 4, 8, 12, and 26 weeks of follow-up. Network meta-analysis was performed using a Bayesian random effects model with non-informative priors. We also assessed the changes in active treatment effects with respect to the type of reference placebo at all time points.
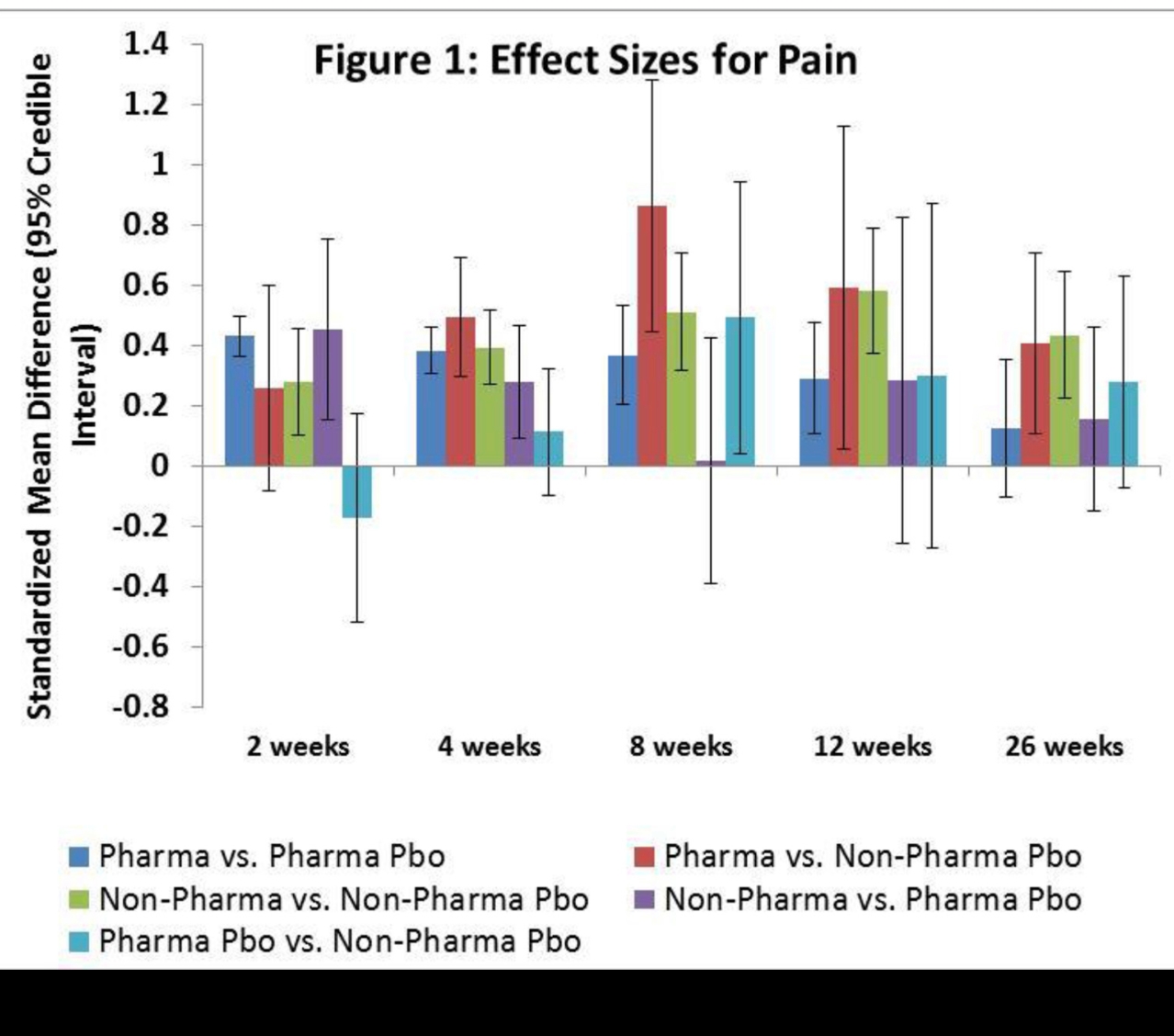
Results: We included 146 trials involving 53,599 participants with knee OA (mean age 61 years, mean BMI 29 kg/m2, mean % female 67%). Placebos used in trials of pharmaceuticals exhibited greater pain improvement than those used in non-pharmaceutical trials at most time points, with the relative effect of pharmaceutical vs. non-pharmaceutical placebo reaching statistical significance at 8 weeks (SMD: 0.49, 95% Credible Interval: 0.05, 0.94) (Fig. 1). When oral pharmaceutical treatments were compared against placebos used in trials of oral non-pharmaceuticals, the effect sizes were greater than those produced in a comparison of oral non-pharmaceutical treatments vs. pharmaceutical placebos at all but one time points.
Conclusions: The results of our network meta-analysis suggest that the placebo effects observed in trials of oral pharmaceuticals are greater than those observed in trials of oral nutraceutical or herbal compounds. The differences observed could be due to variation in patient expectation based on the type of treatment being offered. Smaller placebo effects in trials of non-pharmaceuticals should be heeded as they may inflate treatment effect sizes. With this in mind, these differential placebo effects should be considered by clinicians and patients when interpreting the results of placebo-controlled trials measuring the effectiveness of the respective treatments.