Oral intermittent vitamin D substitution: influence of pharmaceutical form and dosage frequency on medication adherence: a randomized clinical trial
Jean-Pierre Rothen BMC Pharmacol Toxicol. 2020; 21: 51.
Background
To assess adherence to and preference for vitamin D substitution with different pharmaceutical forms and frequencies of administration.
Methods
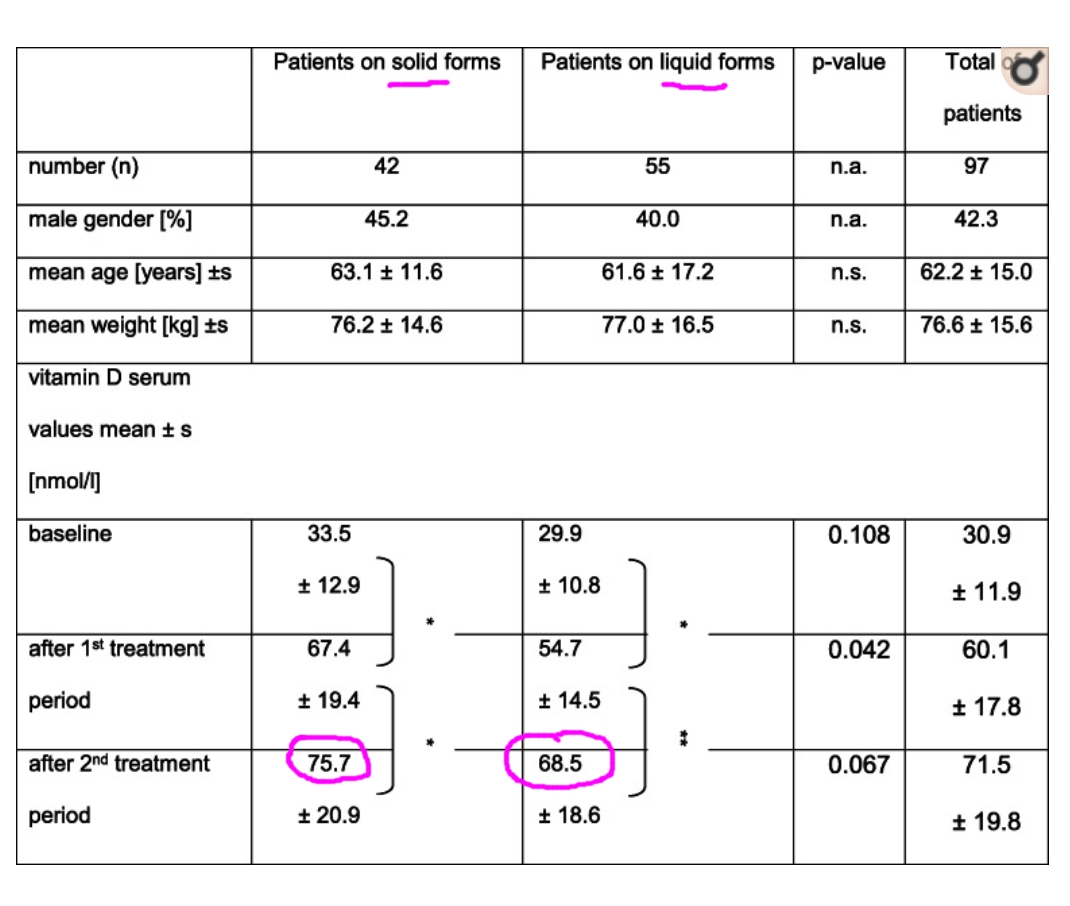
A focus group of stakeholders aimed at preparing the design of an interventional, randomized, cross-over study with 2 × 2 groups obtaining monthly or weekly vitamin D products in liquid or solid form for 3 months each. Dosage corresponds to cumulated amount of recommended 800 IU daily (5.600 IU weekly / 24.000 IU monthly). Main inclusion criteria were a vitamin D serum value < 50 nmol/l and age ≥ 18 years. Primary endpoint was adherence, secondary endpoints were preferences and vitamin D serum levels.
Results
The focus group reached consensus for preference of a monthly administration of solid forms to adults.
Full datasets were obtained from 97 participants. Adherence was significantly higher with monthly (79.5–100.0%) than weekly (66.4–98.1%) administration. Vitamin D levels increased significantly (p < 0.001) in all participants. An optimal value of > 75 nmol/l was achieved by 32% after 3 months and by 50% after 6 months. Preferred formulation was solid form (tablets, capsules) for 71% of participants, and preferred dosage frequency was monthly for 39% of participants.
Conclusions
Monthly oral vitamin D in solid form lead to the highest adherence, and is preferred by the participants. However, only one third of study participants achieved values in the optimal range of > 75 nmol/l cholecalciferol using weekly or monthly administration providing an average daily cholecalciferol dose of 800 IU.