Oral hydrolyzed type 2 collagen protects against the OA of obesity and mitigates obese gut microbiome dysbiosis
S. Soniwala Osteoarthritis and Cartilage VOLUME 26, SUPPLEMENT 1, S173-S174, APRIL 01, 2018
Purpose: While there are no disease modifying therapies to treat OA, numerous nutraceuticals, including glucosamine and chondroitin sulfate, are purported to have joint protective properties. In line with this. we recently reported that hydrolyzed type 1 collagen is joint protective in murine posttraumatic OA (PTOA) and symptom relieving in human OA. However, without insight into mechanism, these findings have been collectively met with skepticism. It has recently been discovered that dysbiosis of the gut microbiome leads to various systemic disease states, with increases in pro-inflammatory microbial species considered pathogenic. In fact, we have reported that high fat diet (HFD)-induced obesity is associated with a gut microbiome dysbiosis that when corrected leads to protection in obesity-accelerated PTOA. It follows that oral nutraceuticals may convey systemic effects and confer protection in OA via alterations in the gut microbiome. Here we report that an orally consumed natural mixture of hydrolyzed type 2 collagen and chondroitin sulfate (hCol2) is joint protective in obesity-accelerated PTOA. Moreover, we provide evidence that hCol2 acts as a prebiotic, altering the gut microbiome in a manner consistent with correction of obesity-associated dysbiosis. These parallel actions of hCol2 may be linked, and support a novel hypothesis that joint protective actions of nutraceuticals stem from previously unappreciated effects on the gut microbiome.
Methods: C57BL/6 mice were fed chow or HFD for 12 weeks, at which point they were provided a daily oral hCol2 supplement. After 2 weeks on supplement, PTOA was induced by DMM surgery. Body mass and glucose tolerance were assessed at various time points pre- and post-DMM, and fecal samples were collected before DMM and at joint harvest 3 weeks post-DMM. 16S rRNA sequencing was performed on fecal extracts to analyze the gut microbiome. Joint tissues were analyzed via standard tissue-based methods including histomorphometric analysis of cartilage structure.
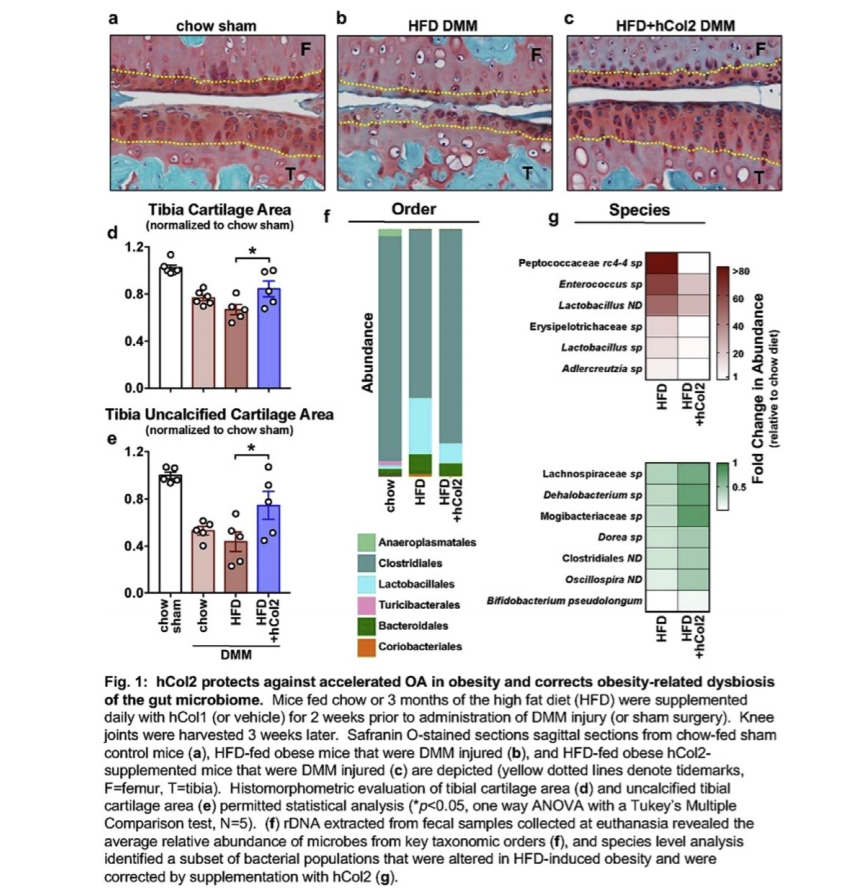
Results: HFD-fed mice became obese, had reduced glucose tolerance, and displayed accelerated PTOA relative to chow-fed mice. While body mass and metabolic phenotypes were not rescued, obese mice consuming the hCol2 supplement had delayed PTOA progression, evidenced by significant preservation of articular cartilage (Fig. 1a-e), with trends toward less cartilage loss than in chow-fed DMM controls (Fig. 1d-e). 16s rRNA sequencing revealed that HFD-induced dysbiosis of the gut microbiome was substantially corrected by hCol2, with the obesity-associated increased abundance of Lactobacillales, Bacteriodales and Coriobacteriales converted to a profile comparable to the chow-fed group (Fig. 1f). Key proinflammatory microbes that were increased in the obese mice, including Peptococcaceae rc4-4 sp. and Enterococcus sp., were reduced following supplementation with hCol2. A series of beneficial microbes were also supported in hCol2-supplemented mice, including Bifidobacterium pseudolongum (Fig. 1g). In aggregate, these effects shifted the obese microbiome to a state more similar to that seen in chow fed mice.
Conclusions: Here we report that dietary supplementation with a novel hCol2 formulation is joint protective in the context of obesity-accelerated PTOA. A parallel a set of effects occur in the gut microbiome, where hCol2 substantially converts the obese microbial profile to that seen in chow-fed mice, with significant suppression of key pro-inflammatory populations that we have previously implicated as pathogenic in the OA of obesity. Given the debate about how nutraceuticals impact the joint and reduce symptoms in OA, we propose the novel concept that hCol2 and agents like it influence joint degeneration indirectly through an alteration in the gut microbiome, begging deeper study of cause and effect in this context.