Histidine Metabolism and Function
Margaret E Brosnan, The Journal of Nutrition, Volume 150, Issue Supplement_1, October 2020, Pages 2570S–2575S,
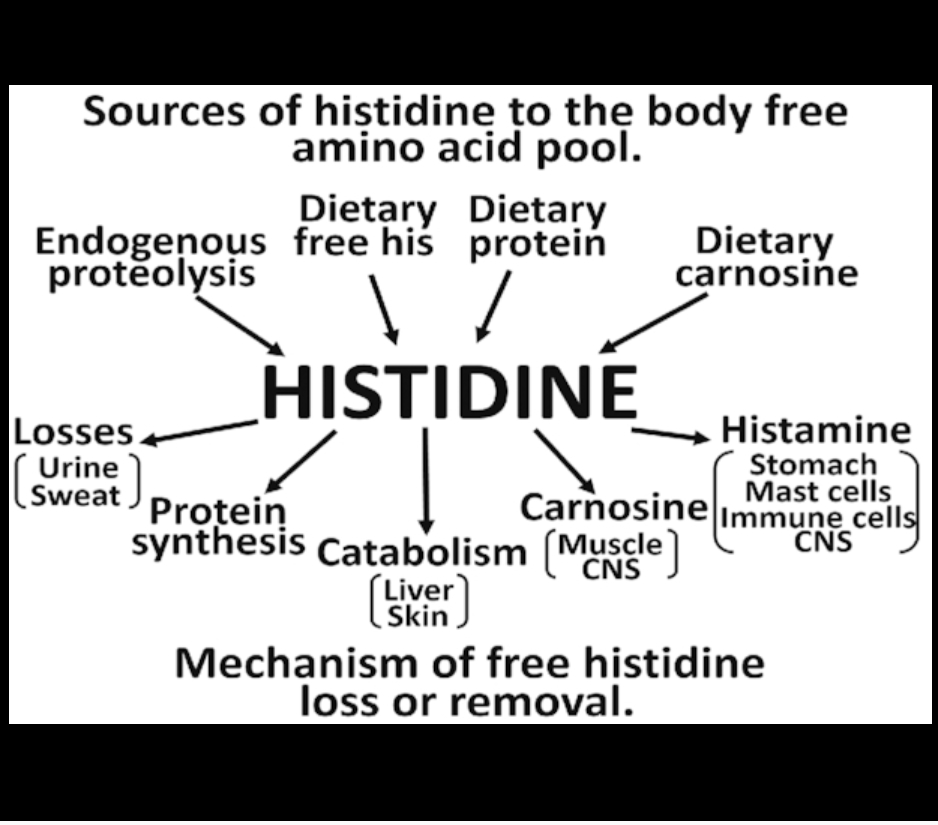
Histidine is a dietary essential amino acid because it cannot be synthesized in humans. The WHO/FAO requirement for adults for histidine is 10 mg · kg body weight−1 · d−1. Histidine is required for synthesis of proteins. It plays particularly important roles in the active site of enzymes, such as serine proteases (e.g., trypsin) where it is a member of the catalytic triad. Excess histidine may be converted to trans-urocanate by histidine ammonia lyase (histidase) in liver and skin.
UV light in skin converts the trans form to cis-urocanate which plays an important protective role in skin. Liver is capable of complete catabolism of histidine by a pathway which requires folic acid for the last step, in which glutamate formiminotransferase converts the intermediate N-formiminoglutamate to glutamate, 5,10 methenyl-tetrahydrofolate, and ammonia. Inborn errors have been recognized in all of the catabolic enzymes of histidine.
Histidine is required as a precursor of carnosine in human muscle and parts of the brain where carnosine appears to play an important role as a buffer and antioxidant. It is synthesized in the tissue by carnosine synthase from histidine and β-alanine, at the expense of ATP hydrolysis. Histidine can be decarboxylated to histamine by histidine decarboxylase.
This reaction occurs in the enterochromaffin-like cells of the stomach, in the mast cells of the immune system, and in various regions of the brain where histamine may serve as a neurotransmitter.