Association between appendicular skeletal muscle index and leukocyte telomere length in adults: A study from National Health and Nutrition Examination Survey (NHANES) 1999–2002
Lingzhi Chen Clinical Nutrition Volume 40, Issue 5, May 2021, Pages 3470-3478
Background
A higher body mass index (BMI) is associated with shorter telomeres. The loss of muscle mass with aging is associated with adverse outcomes. The appendicular skeletal muscle index (ASMI) is currently used to quantify muscle mass.
Objective
We investigated the association of the ASMI with leukocyte telomere length in adult Americans.
Methods
This cross-sectional study used the National Health and Nutrition Examination Survey (NHANES) 1999–2002 dataset. Body composition was measured by dual-energy X-ray absorptiometry. Low muscle mass was defined using sex-specific thresholds of the appendicular skeletal muscle mass index (ASMI). The telomere-to-single-copy gene ratio (T/S ratio) was converted to base pairs. Generalized linear models were performed to evaluate the association of ASMI with telomere length.
Results
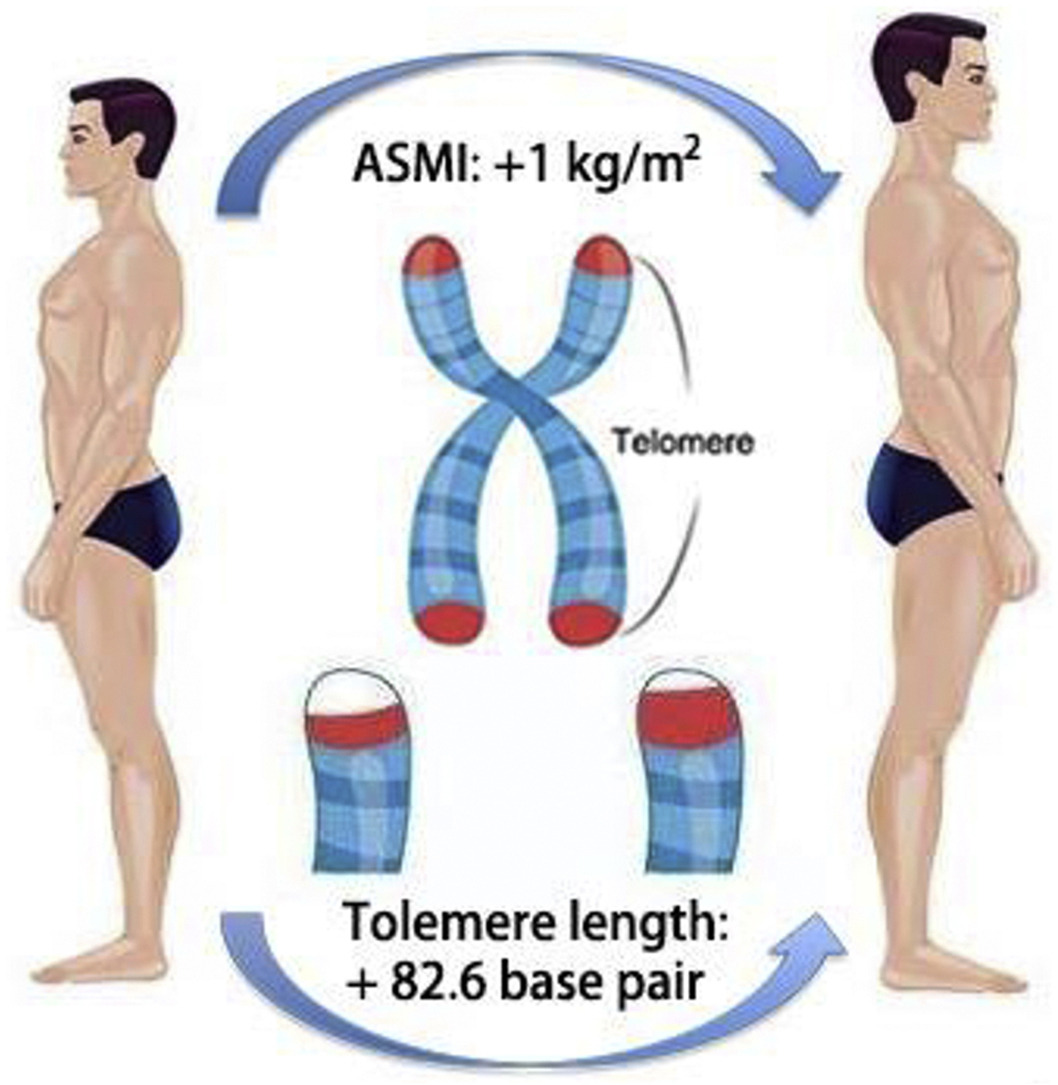
In multivariable adjustment regression models, higher ASMI was associated with longer telomeres in US adults (β = 70.2, P < 0.001, P trend<0.001). In participants with preserved muscle mass, the ASMI was related to longer telomere length (β = 75.1, P < 0.001), but not significantly in low muscle mass participants (β = 68.7, P = 0.30). Further subgroup analysis by a combination of age groups and muscle mass status showed positive association with young-preserved muscle mass (β = 82.6, P < 0.001), old-preserved muscle mass (β = 44.4, P = 0.12), young-low muscle mass (β = 135.4, P = 0.20), and old-low muscle mass (β = 52.7, P = 0.55). Because each additional year of chronological age was associated with telomeres that were 15.3 base pairs shorter, on average, this would equate to 5.4 fewer years of biological aging (82.6 ÷ 15.3) in the young-preserved muscle mass participants.
Conclusions
A higher ASMI is associated with longer telomeres. The prevention of skeletal muscle loss has the potential to delay telomere shortening and account for less biological aging.