Oxidative stability of omega-3 tablets
Tina Lien Vestland European Journal of Lipid Science and Technology Volume 119, Issue 2 February 2017
Preparing solid formulations, as powder and tablets, containing omega-3 can be challenging as the necessary production processes expose the unsaturated omega-3 fatty acids to high temperatures, light, and mechanical stress in presence of air. The present work demonstrates that omega-3 tablets can be prepared with sufficiently low total oxidation values (totox) to satisfy relevant monographs for omega-3 products. The tablets were prepared from spray granulated direct compaction grade 30% w/w triglyceride powders as characterized by Vestland, Jacobsen, Sande, Myrset, and Klaveness, 2015 (Food Chem. 2015. 185: p. 151–158; Food Chem., 2016. 197, Part A: p. 496–502). Addition of ascorbic acid, in combination with EDTA as processing agent, was correlated with lower totox in powders. Spray granulation performed under nitrogen atmosphere contributed to significantly decreased totox in powders after 8 months of storage at accelerated temperature (37°C) compared to spray granulation in air. In long-term stability studies, it was confirmed that coated omega-3 tablets remained at totox <5 after 1 year of storage at ambient temperature.
Practical applications: The confirmation that oxidative stable omega-3 tablets can be produced opens the possibility for a new administration form in the omega-3 field. The use of dry powders in the production process imply more opportunities within combination products as dry powders typically exhibit fewer compatibility issues. Omega-3 tablets are a gelatin- and lactose-free alternative for health conscious individuals all over the world.
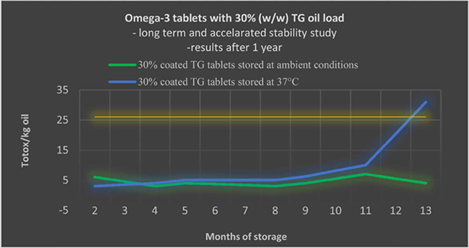
The most frequently used formulation form for health supplements is tablets, however, to date there have been numerous issues with achieving sufficient oil load and maintain oxidative stability trying to formulate omega-3 oil in tablets. Totox 26 is the limit set by the Global Organization for EPA and DHA (GOED) for omega-3 supplement for human use (yellow line). As presented in this figure, omega-3 tablets with 30% w/w triglyceride oil load (60% concentrate) have been prepared and totox have maintained lower than 26 for tablets kept at 37°C for 1 year. The data so far obtained from the long-term stability study (tablet samples kept at ambient conditions) indicate the possibility for a +3 years shelf life for the omega-3 tablets.













