Dietary Supplements for Treating Osteoarthritis: a Systematic Review and Meta-analysis
X. Liu Osteoarthritis and Cartilage VOLUME 25, SUPPLEMENT 1, S292-S293, APRIL 01, 2017
Purpose: Although widely used by patients with osteoarthritis, the efficacy and safety of dietary supplements remains unclear and is often clouded by misinformation in mainstream media. The aim of this study is to conduct a systematic review and investigate the efficacy and safety of all dietary supplements compared with placebo for patients with osteoarthritis.
Methods: MEDLINE, EMBASE, CENTRAL, AMED, and CINAHL were searched from inception to December 2015. Randomized controlled trials comparing oral dietary supplements with placebo for hand, hip or knee osteoarthritis were eligible for inclusion. The search was limited to English-language publications. The review processes were conducted independently by two reviewers. Primary outcomes included pain and physical function. Secondary outcomes were stiffness, radiograph, analgesic use, quality of life, and adverse effects. Data was pooled using a random-effects model. Cohen’s criteria were used to interpret the estimated treatment effects as small, moderate or large. The threshold for minimum clinically important difference was 0.37 standardized units. The GRADE approach was used to assess the quality of evidence.
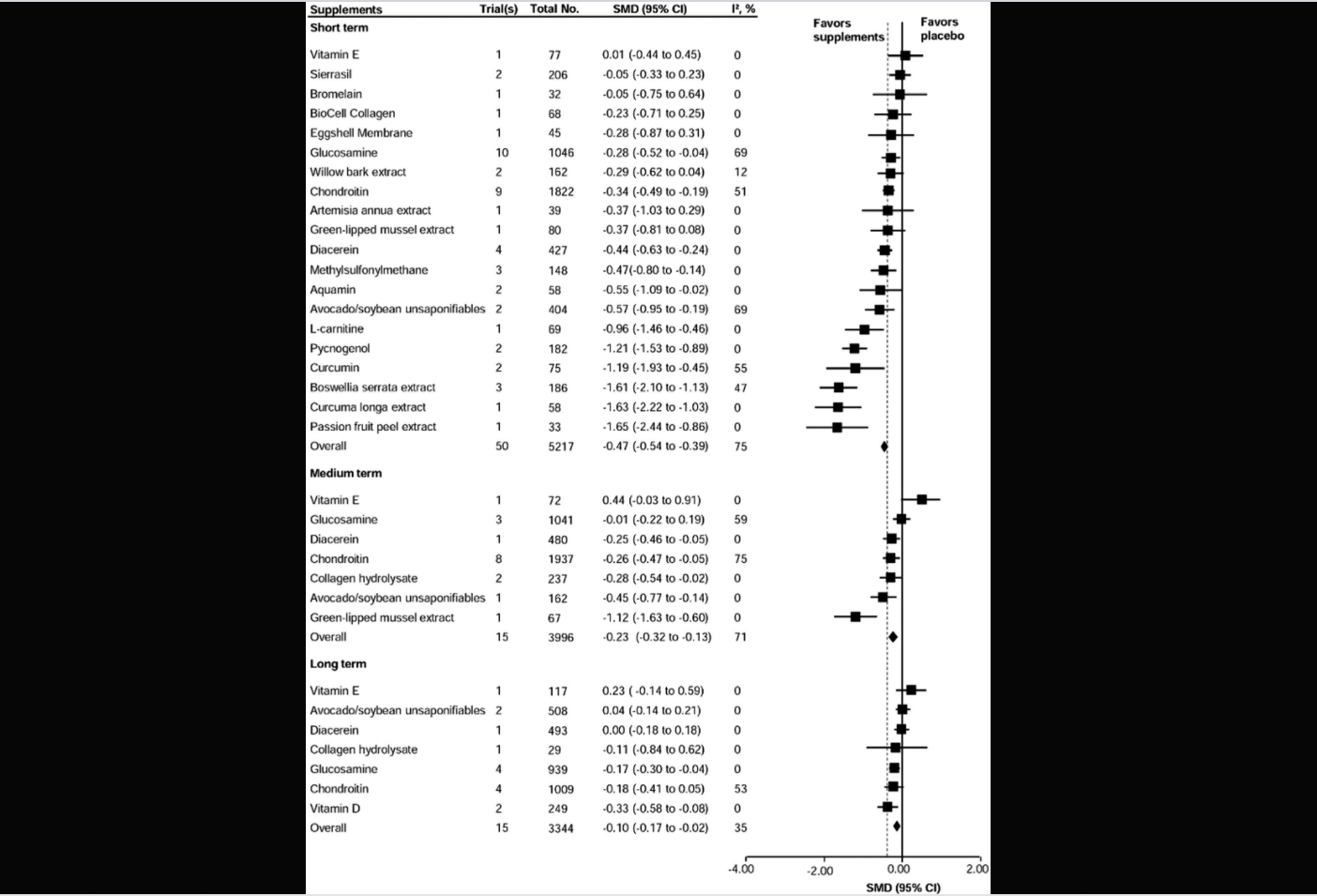
Results: Seventy-one randomized placebo-controlled trials with 11,021 participants investigating 24 dietary supplements were included. Of the 24 supplements investigated in this review, six (passion fruit peel extract, curcuma longa extract, boswellia serrata extract, curcumin, pycnogenol and L-carnitine) demonstrated large (effect size >0.80) and clinically important effects for pain reduction at short-term. Another six supplements (avocado soybean unsaponifiables, aquamin,methylsulfonylmethane, diacerein, glucosamine and chondroitin) revealed statistically significant improvements on pain, but were of unclear clinical importance. Only green-lipped mussel extract had clinically important effects on pain at medium-term. No supplements were identified with clinically important effects on pain reduction at long-term (See the Figure overleaf). Similar results were found for physical function. Chondroitin demonstrated statistically significant, but not clinically important structural improvement (effect size −0.30, −0.42 to −0.17). There were no differences between supplements and placebo for safety outcomes, except for diacerein. The GRADE assessment suggested a wide range of quality evidence from very low to high.
Conclusions: Some supplements demonstrated large treatment effects for osteoarthritis despite the wide range of quality of evidence, while widely used supplements were either ineffective or showed small and arguably clinically unimportant treatment effects. Our review should be used to inform clinical decision making in this contentious area.