Zinc and the modulation of Nrf2 in human neuroblastoma cells
Z.Kaufman Free Radical Biology and Medicine Volume 155, 1 August 2020, Pages 1-9
Highlights
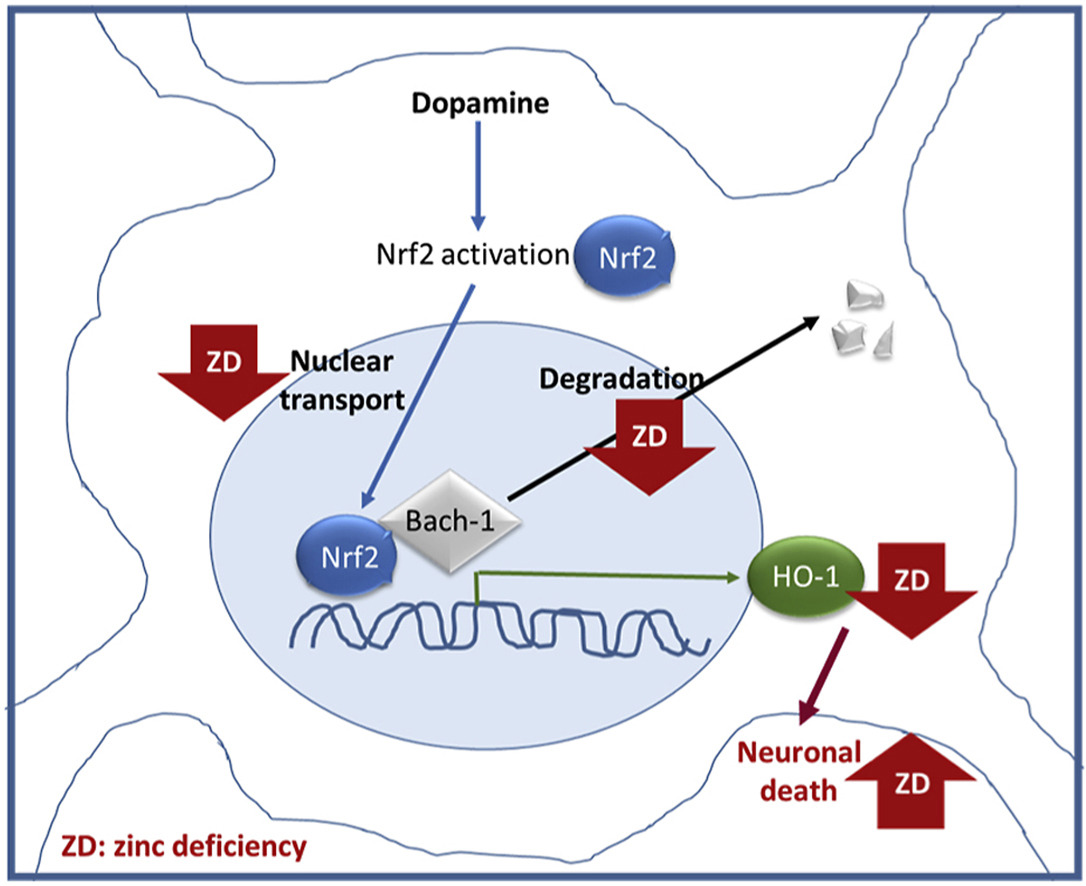
• Dopamine (DA) neurotoxicity is exacerbated by zinc deficiency.
• Zinc deficient cells show an impaired DA-induced HO-1 upregulation.
• A low zinc availability leads to tubulin oxidation and decreased Nrf2 nuclear transport.
• A low zinc availability inhibits the degradation of the Nrf2 repressor, Bach-1.
• Zinc is crucial in the neuronal response to DA-induced oxidative stress.
Zinc plays a key role in the modulation of neuronal redox homeostasis. A decreased zinc availability is associated with neuronal NADPH oxidase and nitric oxide synthase activation, deregulation of redox signaling, and impaired glutathione synthesis. The present work tested the hypothesis that zinc is necessary in the neuronal defense response against dopamine (DA)-induced oxidative stress, in particular through heme oxygenase-1 (HO-1) upregulation. DA showed higher cytotoxicity when zinc availability was low. Human IMR-32 neuroblastoma cells responded to high DA concentrations (100 μM) by upregulating HO-1. This upregulation involved Nrf2 translocation to the nucleus, degradation of the Bach-1 repressor, and Nrf2-DNA binding, but it was independent of ERK1/2 activation. DA-mediated induction of HO-1 expression was dependent on the concentration of zinc in the medium. IMR-32 cells incubated in zinc deficient medium showed an impaired response to DA, with lower HO-1 mRNA and protein levels than control DA-challenged cells. This altered HO-1 upregulation was reversed by zinc supplementation. In the presence of DA, Nrf2 nuclear translocation and Bach-1 degradation were lower in zinc deficient cells. The mechanisms involved include: i) impaired Nrf2-tubulin interactions and ii) alterations in the proteasome-mediated degradation of Bach-1 secondary to a decreased ubiquitylation. Results suggest that zinc is crucial in the neuronal response to DA-induced oxidative stress in part through its role in the modulation of the Nrf2-and Bach-1-driven upregulation of HO-1 expression.