UVA irradiation increases ferrous iron release from human skin fibroblast and endothelial cell ferritin: Consequences for cell senescence and aging
Matthew J.Smith Free Radical Biology and Medicine Volume 155, 1 August 2020, Pages 49-57
Highlights
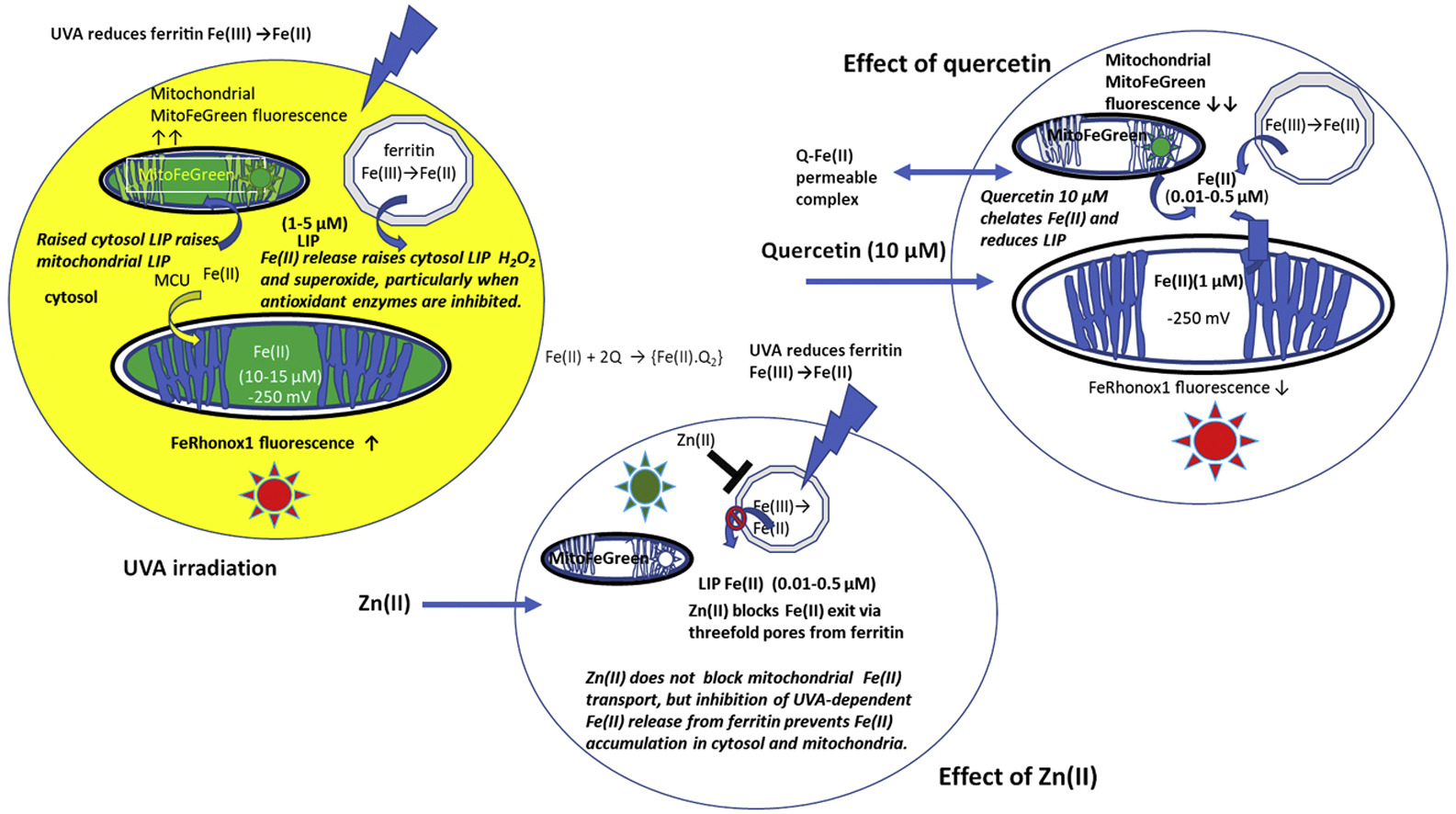
• UVA irradiation causes immediate increases in cytosolic and mitochondrial Fe(II) in human dermal fibroblasts and endothelial cells.
• UVA irradiation increases dermal endothelial H2O2, monitored using the adenovirus vector Hyper-DAAO-NES.
• UVA-dependent increases in Fe(II) and H2O2 are inhibited by ZnCl2 (10 μM) via ferritin's 3-fold iron conducting channels.
• Quercetin reduces UVA dependent Fe(II) signals and H2O2 production.
• Inhibition of superoxide dismutase and catalase increases and prolongs the cytosolic Fe(II) signal after UVA irradiation.
• UVA-dependent ferritin iron release is the most proximal cause of UV-dependent dermal inflammation.
UVA irradiation of human dermal fibroblasts and endothelial cells induces an immediate transient increase in cytosolic Fe(II), as monitored by the fluorescence Fe(II) reporters, FeRhonox1 in cytosol and MitoFerroGreen in mitochondria. Both superoxide dismutase (SOD) inhibition by tetrathiomolybdate (ATM) and catalase inhibition by 3-amino-1, 2, 4-triazole (ATZ) increase and prolong the cytosolic Fe(II) signal after UVA irradiation. SOD inhibition with ATM also increases mitochondrial Fe(II). Thus, mitochondria do not source the UV-dependent increase in cytosolic Fe(II), but instead reflect and amplify raised cytosolic labile Fe(II) concentration. Hence control of cytosolic ferritin iron release is key to preventing UVA-induced inflammation. UVA irradiation also increases dermal endothelial cell H2O2, as monitored by the adenovirus vector Hyper-DAAO-NES(HyPer). These UVA-dependent changes in intracellular Fe(II) and H2O2 are mirrored by increases in cell superoxide, monitored with the luminescence probe L-012. UV-dependent increases in cytosolic Fe(II), H2O2 and L-012 chemiluminescence are prevented by ZnCl2 (10 μM), an effective inhibitor of Fe(II) transport via ferritin's 3-fold channels. Quercetin (10 μM), a potent membrane permeable Fe(II) chelator, abolishes the cytosolic UVA-dependent FeRhonox1, Fe(II) and HyPer, H2O2 and increase in MitoFerroGreen Fe(II) signals. The time course of the quercetin-dependent decrease in endothelial H2O2 correlates with the decrease in FeRhox1 signal and both signals are fully suppressed by preloading cells with ZnCl2.
These results confirm that antioxidant enzyme activity is the key factor in controlling intracellular iron levels, and hence maintenance of cell antioxidant capacity is vitally important in prevention of skin aging and inflammation initiated by labile iron and UVA.