The COVID-19 pandemic: is there a role for magnesium? Hypotheses and perspectives
Magnesium Research Volume 33, issue 2, April-May-June 2020 Stefano Iotti
In the last 20 years, three zoonotic epidemics – severe acute respiratory syndrome (Sars) in 2003, Middle East respiratory syndrome (Mers) in 2012, and, since December 2019, Covid-19 – were triggered by β-coronaviruses (CoV) and exacted a high toll in human lives [1-3]. Responsible for Covid-19 is SARS-CoV-2, which shares a high degree of homology with SarsCoV and MersCoV [4].
β-CoV belong to the large family of Coronaviridae, single-stranded RNA viruses. About 70% of their genome codes for replicases/transcriptases, which are crucial for viral replication, while the remaining 30% codes for structural proteins, i.e., spike (S), membrane (M), nucleocapside (N), and envelope (E) proteins. The S-proteins, responsible for the crown-like shape of the viruses, play a crucial role in pathogenesis of the diseases since they engage host proteins to infect the cells. While the S protein of MersCoV binds to human DiPeptidyl Peptidase 4 [5], the S proteins of SarsCoV and SARS-CoV-2 bind with similar affinity to the angiotensin converting enzyme (ACE)2 [3, 6], ubiquitously expressed and rather abundant in the lung, heart, kidney, and blood vessels [7]. The S proteins need to be primed by host proteases, among which transmembrane protease-serine (TMPRSS)2, to allow viral entry into the cells [8]. It is noteworthy that the S protein of SARS-CoV-2 has also a potential cleavage site for furin [9], a calcium-dependent serine endoprotease that is abundant in the lung (https://www.proteinatlas.org/ENSG000001 ... RIN/tissue). This furin binding site has been described in highly pathogenic viruses and might augment SARS-CoV-2's internalization.
After being inhaled, SARS-CoV-2 reaches the nose and the throat, where it infects the epithelial cells, which are rather rich in ACE2. In the initial stages of the infection, there are either no symptoms or mild clinical manifestations, such as dry cough, sore throat, mild fever, smell and taste dysfunction, and general malaise. If the immune system fails in controlling the infection in the early stages, the virus reaches the alveoli, lined by cells that express high levels of ACE2, and interstitial pneumonia develops. In about 5% of the patients, a fatal and fulminant hypercytokinaemia dramatically and rapidly deteriorates the clinical conditions with the onset of acute respiratory distress, thromboembolism, and multiple organ failure [10-12] (figure 1).
Accordingly, the anti-IL6 Tocilizumab and the IL1-receptor antagonist Anakinra have been approved to treat patients with COVID-19. As of May 2, COVID-19 is considered a respiratory infection with important systemic effects that importantly impact the immune and cardiovascular systems. An early and prognostic lymphopenia occurs in more than 80% of patients, with a more significant reduction of CD4+ than CD8+ [13-15].
In addition, endothelial cell involvement has been shown in various vascular beds in patients with COVID-19 [16]. SARS-CoV-2 can directly infect endothelial cells using the ACE2 receptor. Moreover, the cytokine storm triggered by the overwhelming inflammatory response to the virus impairs endothelial function, thus increasing permeability, inducing vasoconstriction, and fostering thrombogenesis [14]. These detrimental events in the lung microvasculature greatly imbalance the ventilation/perfusion ratio, rapidly leading to acute respiratory failure, while in other organs SARS-CoV-2-associated endothelial dysfunction causes ischemia and organ failure. Indeed, a recent paper in JAMA reports that “COVID-19 is a systemic disease that primarily injures the vascular endothelium” [17].
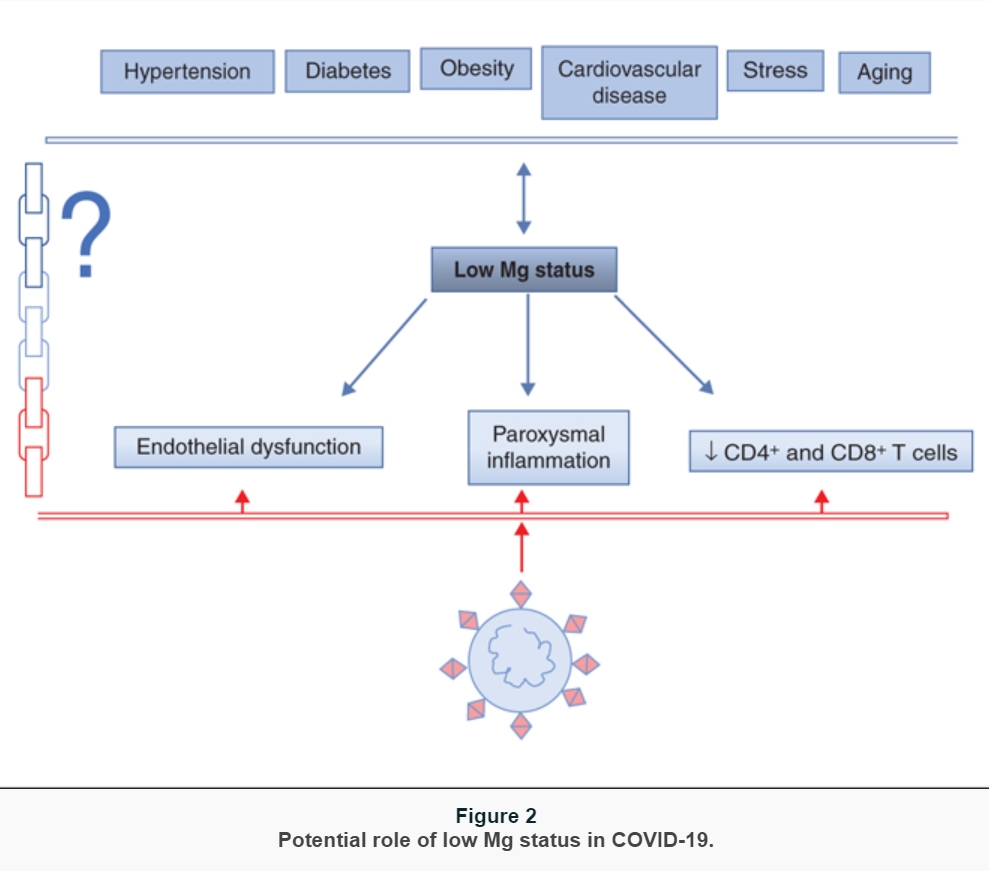
While learning about the clinical presentation and the pathophysiology of COVID-19, it became clear that some features of the disease recall the symptoms and signs that are described in magnesium (Mg) deficiency [18]. Therefore, it is feasible to hypothesize that Mg deficiency, rather common in western world because a large part of population does not consume adequate amounts of Mg [19], might contribute to the onset, progress, and severity of COVID-19. To our best knowledge, at the moment there are no data about Mg homeostasis in COVID-19, which is not surprising because magnesemia is not routinely evaluated in clinical practice. On the other hand, it should be stressed that the severe Mg deficiency with clinical symptoms nowadays is rare. Rather, there is the occurrence of a latent subclinical Mg deficiency that is difficult to detect by routine laboratory evaluation of Mg in serum. Here, we list some cues that might be useful for challenging discussions and future studies (figure 2):
individuals with comorbidities, i.e., hypertension, cardiovascular diseases, diabetes, and obesity, are more prone to develop severe COVID-19. These diseases are all characterized by hypomagnesemia which might be exacerbated by some pharmaceuticals (diuretics, proton-pump inhibitors), and the supplementation of Mg has beneficial effects [20]. Latent Mg deficiency is associated with chronic low-grade inflammation. Indeed, a meta-analysis and systematic reviews indicate that dietary Mg intake is significantly and inversely associated with serum C reactive protein (CRP) levels [21]. Accordingly, Mg supplementation reduces CRP levels in individuals with inflammation (CRP levels > 3 mg/dL) [22].
In a recent study, it has been shown that Mg supplementation attenuates disease severity and accelerates recovery in experimental murine colitis [23].Another hint comes from the evidence that COVID-19 is particularly severe and associated with high mortality in the elderly [24], known to be frequently Mg deficient because of malnourishment, comorbidities, and polypharmacy. Another puzzling issue to consider is the relation existing between Mg and stress. There is no doubt that the SARS-CoV-2 pandemic has generated stress not only among health professionals but also in common people, because of confinement, fear, economic uncertainty. Stress hormones, i.e., catecholamines and corticosteroids, cause the shift of Mg from the intracellular to the extracellular space which may result in enhanced urinary excretion of Mg and consequent reduction of serum Mg, which, in turn, increases the release of catecholamines, adrenocorticotrophic hormone, and cortisol, thus creating a vicious circle of reduced resistance to stress and further Mg depletion [25, 26] ;
Mg plays a role in shaping innate and adaptive immune systems [18]. A low Mg status activates inflammation, by sensitizing sentinel cells to the noxious agent, priming phagocytes and participating to the orchestration of the vascular and cellular events that characterize the process [27]. In in vivo models, a drop of magnesemia results in the classical inflammatory response, characterized by hyperemia, edema and a significant increase of the plasma levels of IL-6 and acute phase proteins [27]. Interestingly, evidence has been provided that Mg concentration in acutely inflamed tissues is reduced through the activation of the IL-33/ST2 axis [28]. On these bases, we hypothesize that a subclinical Mg deficiency exacerbates virus-induced inflammation, which determines a fall of local Mg, thus fostering an uncontrolled release of high amounts of pro-inflammatory cytokines. The final result is the onset of a cytokine storm that can be fatal. Turning our attention to adaptive immune system, it is notable that in vitro and in vivo the proliferation and the activation of both CD4+ and CD8+ T lymphocytes are greatly reduced in Mg-deficient conditions [29]. Even more interesting is the evidence that CD8+ and, to a lower extent, CD4+ T cells are significantly reduced in the lung of Mg-deficient mice after inhalation of influenza A virus, resulting in exacerbated morbidity [29]. CD8+ and CD4+ impairment reported in COVID-19 might be, in part, supported by a low Mg status;
Mg is important in maintaining endothelial function and, therefore, vascular integrity [30]. Mg deficiency induces a pro-inflammatory phenotype, which means increased release of chemokines and cytokines as well as increased thrombogenicity. In response to inflammatory stimuli, the endothelium releases ultra-large multimers of von Willebrand factor, which form high-strength bonds with the platelets, thus favoring their binding to the arterial wall [30]. In parallel, Mg deficiency promotes platelet aggregation and their release of beta thromboglobulin and thromboxanes [31]. In addition, low Mg also affects endothelial fibrinolytic activity by upregulating type 1 plasminogen activator inhibitor and preventing plasmin formation [30]. All together, these findings underpin that systemic or local Mg deficiency predicts platelet-dependent thrombosis. At the present moment, it is not clear whether SARS-CoV-2 alters endothelial function by directly infecting them and/or through the inflammatory response [32]. However, it is clear that a subtle chronic Mg deficiency might create a favorable microenvironment for the virus to promote thromboembolism;
Mg also maintains proper lung function and reduces the risk of airway hyper-reactivity and wheezing [33]. This is a relevant issue in respiratory tract infections. In addition, Mg reduced the release of TGFβ1 thereby preventing the deposition of collagen and consequently lung fibrosis in vivo[34]. Fibrosis is a disabling outcome of interstitial lung diseases. In some patients who recovered from COVID-19, lung fibrosis may develop [35] and Mg might be beneficial.
If Mg status impacts the susceptibility and the response to SARS-CoV-2 as depicted above, it is legitimate to hypothesize that Mg intake might influence COVID-19 outbreak. This would represent the typical task for a large-scale epidemiological study employing meta-analysis approach, which, however, could be better performed when the COVID-19 global outbreak is almost halted and the data of global deaths and confirmed cases of infection are more accurate. Meanwhile, as a work hypothesis, we perform a sort of preliminary survey employing the current official data of COVID-19 outbreak and the available data present in the literature on Mg intake. A clever approach often employed to evaluate the Mg intake in the US population is to refer to the map of water hardness of USA [36], exploiting the general habit of the US people to drink tap water. This approach of using the water hardness figure to explore a possible link between Mg and some diseases has been used in several studies reported in the literature, in particular to correlate the level of Mg, which is higher in hard water, and the risk of cardiovascular diseases [37]. Based on this approach, we focused on Colorado as state reference since it has the peculiarity of being an isolated area having a low-average water hardness (and therefore indicating a lower Mg intake (90-180 ppm) within a large area with hard water States (> 180 ppm) [36]. Therefore, the ratio of the comparison of the COVID-19 outbreak is taking the State Colorado as a model, comparing its outbreak data with the seven surrounding states: Utah, New Mexico, Kansas, Oklahoma, Arizona, Wyoming, Nebraska. Outbreak data were taken from https://www.vox.com/2020/3/26/21193848/ ... s-by-state at two different dates and are reported in table 1. Many more deaths and confirmed cases of infected people were reported in Colorado than in all the surrounding states. These differences are even more evident if we consider the data of the number of “tests per million people” (last right column). Our analysis is only suggestive and has no claim of completeness, since we did not consider other relevant factors such as population mobility in the period considered and many other variables that could have influenced the outbreak. We need and look forward to a rigorous meta-analysis that, as discussed above, could be performed in the terminal phase of COVID-19 outbreak.
CONCLUSIONS
Mg is the forgotten cation. However, it is well documented that low Mg dietary intake, several pathologies or drugs that impair its homeostasis – among which the widely used proton pump inhibitors and thiazides – lead to an impairment of Mg homeostasis, which is associated with important health problems [20].
There are common cues in Mg deficiency and COVID-19 that suggest the relevance of measuring magnesemia in all the patients in the different stages of the disease and, in case of deficiency, of supplementing the cation. A correct level of Mg serum levels might also represent an effective and inexpensive preventive countermeasure against the virus. Last, Mg supplementation might reveal very useful in managing the stress triggered by the pandemic as well as the posttraumatic stress disorder that will plague COVID-19's survivors, health professionals, and common people who have to face important changes of their habits and life-style.
Needless to say, more basic, translational, and clinical research is required to underpin the potential relationships between Mg status and COVID-19.