Role of Vitamin D in COVID-19: Active or Passive?
Bess Dawson-Hughes The Journal of Clinical Endocrinology & Metabolism, Volume 106, Issue 12, December 2021, Pages e5260–e5261
Amidst a raging pandemic, Nogues et al. developed and implemented a protocol to determine whether treatment with calcifediol compared with no calcifediol altered the course of 838 coronavirus disease 2019 (COVID-19) patients admitted to a hospital in Barcelona, Spain (1). This was not a classical randomized, controlled trial, but rather a real-world examination of outcomes of patients assigned (on a bed availability basis) to 1 of 8 COVID wards, 3 of which had chosen not to administer calcifediol and 5 that had chosen to do so. Other practices in the 8 wards were standardized. Some patients did not have serum 25-hydroxyvitamin D (25(OH)D) measurements upon admission for reasons related to staff availability. The supplemented patients had significantly fewer transfers to the intensive care unit (adjusted odds ratio, 0.13 [95% CI, 0.07-0.23]) and lower mortality rates (adjusted odds ratio, 0.52 [95% CI, 0.27-0.99]) than the unsupplemented patients, findings that have important implications for the in-hospital management of COVID-19 patients globally.
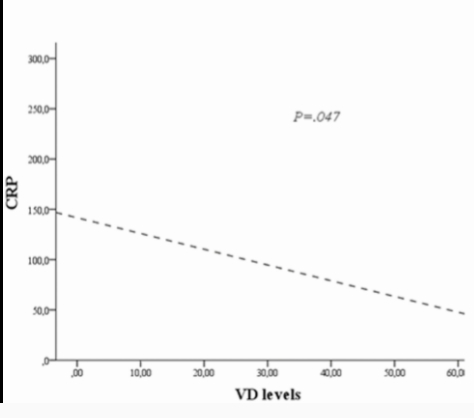
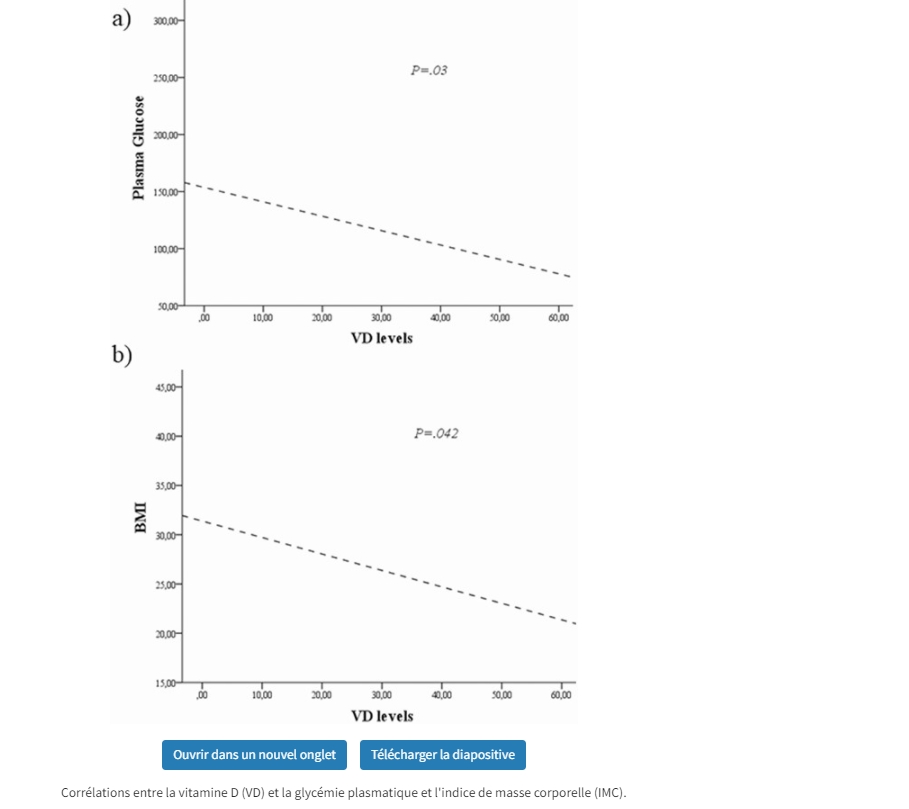
25(OH)D was measured in 678 of the 838 patients upon admission to the hospital. It was notable that the median 25(OH)D level was 13 [interquartile values 8, 24] ng/mL in the calcifediol-treated group and 12 [8, 19] ng/mL in the untreated control group. How might we interpret the significance of these very low 25(OH)D levels at the time of hospitalization? Was low 25(OH)D a predisposing factor to serious COVID-19 infection or was it a marker of inflammation associated with acute illness? The answer has implications for patient care.
A Predisposing Factor
The patients’ values, measured in March through May 2020, were far lower than those expected in the general population, even for the early spring, when levels tend to be lowest in the Northern hemisphere. Barcelona has a similar latitude to Boston (41.4 vs 42.4 degrees N). In a cross-sectional study of older postmenopausal women not taking vitamin D supplements, mean 25(OH)D levels in Boston in March, April, and May were 22, 23, and 28 ng/mL, respectively (2). These values are consistently higher than the median levels of 12 and 13 ng/mL reported by Nogues (1). Although the assays used by Nogues and Krall were not standardized to the same reference material, the values that Nogues et al. documented are much lower than expected, consistent with the concept that COVID-19 severe enough to require hospitalization occurred more frequently in the segment of the Barcelona population with low rather than representative 25(OH)D values. Although relevant evidence is inconsistent (3, 4), it is plausible that vitamin D deficiency and insufficiency were a risk factor for severe COVID-19 infection requiring hospitalization.
A Marker of Severe Illness
An alternative explanation of the very low 25(OH)D values reported by Nogues et al. is worth considering. The patients may have had 25(OH)D levels that were representative of those in the Barcelona area before contracting COVID-19, but their COVID-19 illness caused these levels to decline precipitously. Acute illness with high levels of inflammation has been associated with very low 25(OH)D levels in several studies, including one conducted in Barcelona before the COVID-19 pandemic (January-November 2015), in which 135 patients were admitted to a university hospital intensive care unit for a variety of illnesses. These patients had a mean 25(OH)D level of 11 ng/mL (range, 7-20 ng/mL) (5), and nonsurvivors had significantly lower 25(OH)D levels than survivors (median, 8.1 ng/mL [interquartile values 6.2, 11.5] vs 12.0 ng/mL [7.1, 20.3].
The robust positive response to supplementation observed by Nogues et al. would seem unlikely in patients who had low 25(OH)D levels solely on the basis of critical illness and is consistent with the notion that vitamin D deficiency/insufficiency is a contributing factor in the development and progression of COVID-19. If so, this would support the rationale not only for supplementation during hospitalization, but also for avoiding vitamin D deficiency, as a safe and low-cost measure to prevent COVID-19 infection.
A specific COVID-19-related guideline for vitamin D intake is premature; however, it does seem prudent during the pandemic to adhere to current vitamin D intake recommendations with greater attention and urgency. The National Academy of Medicine recommendation of 800 IU per day of vitamin D for older adults is based on evidence that it benefits the skeleton (6), but evidence is expanding that this level of intake may also reduce risk of infection. In an individual participant data meta-analysis of trials conducted before the COVID-19 pandemic, Martineau et al. found that daily or weekly doses of vitamin D reduced risk of acute respiratory infections, with doses < 800 IU/d lowering risk by 20% and doses of 800 to 2000 IU/d lowering it similarly, by 19% (7). Moreover, the benefit of supplementation appeared to be greater among those with 25(OH)D levels below 20 ng/mL than in those with higher 25(OH)D levels. The work of Nogues et al. extends current evidence, by adding COVID-19 to the list of infections that are likely mitigated by maintaining an optimal vitamin D status. Finally, as previously noted (8), the favorable effects of vitamin D on bone and muscle provide a strong rationale for maintaining vitamin D adequacy after hospital discharge in COVID-19 survivors who face an arduous rehabilitation process.