par Nutrimuscle-Conseils » 6 Jan 2022 13:12
par Nutrimuscle-Conseils » 6 Jan 2022 13:12
Role of the T cell vitamin D receptor in severe COVID-19
Jay K. Kolls Nature Immunology volume 23, pages5–6 (2022)
New research provides evidence of an impaired vitamin D gene signature in CD4+ T cells in patients with severe COVID-19. Mechanistically, it is shown that vitamin D alters the epigenetic landscape of CD4+ T cells, as well as inducing key transcription factors such as STAT3, BACH2 and JUN that reduce levels of IFN-γ and increase IL-10. These changes generate pro-resolving TH1 cells that may be beneficial in resolving or preventing severe COVID-19.
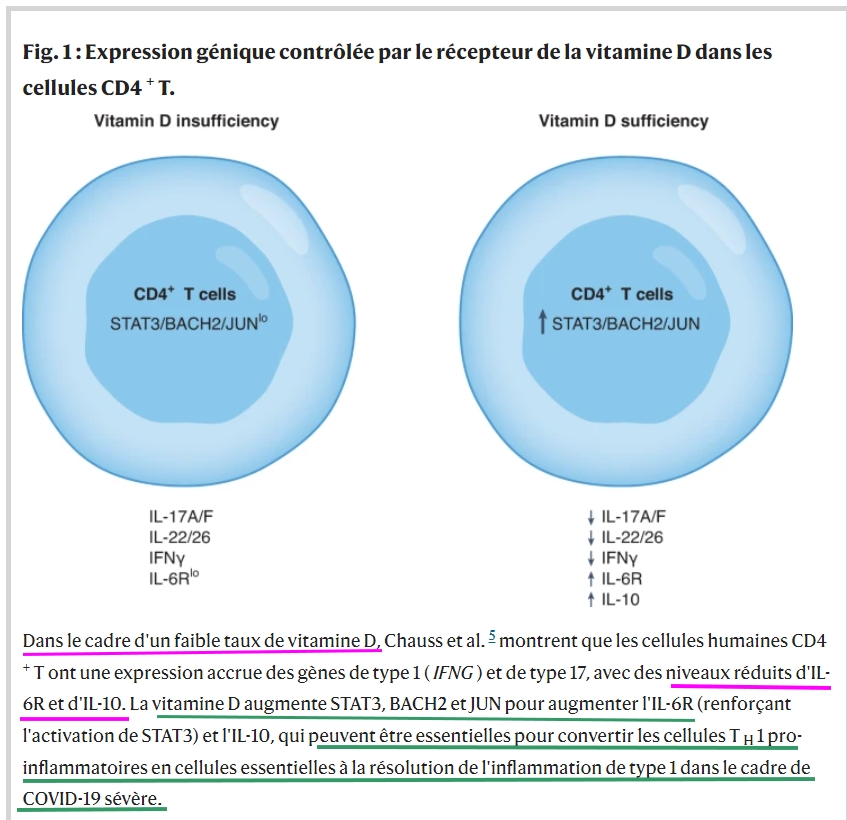
As of November 2021, SARS-CoV-2, the cause of the COVID-19 pandemic, has infected more than 250 million people worldwide. Infections can be asymptomatic or lead to severe acute respiratory distress syndrome (ARDS), which can be fatal. Host factors that attribute to this spectrum of illness include male sex, obesity, diabetes, hypertension and microbiome status, as well as nutritional status1,2. It is well documented that severe COVID can arise due to an insufficient immune response, such as that which may occur in immunocompromised individuals3. Severe COVID-19 also occurs in non-immunosuppressed individuals and when the immune response is too vigorous, resulting in a cytokine storm4. In this issue of Nature Immunology, Chauss and colleagues5 provide new data showing that severe COVID-19 in some individuals may result from a failure to resolve an exuberant type I immune response, and specifically implicate vitamin D receptor (VDR) signaling in this process.
Dexamethasone—a potent anti-inflammatory steroid—improves outcomes in COVID-19-associated ARDS, but has no effect or can even worsen ARDS caused by other triggers. To approach this conundrum, the authors mined single-cell RNA sequencing (RNA-seq) data from the lungs of patients with COVID-19 for comparison with control samples. They found upregulation of STAT1, TBX21, IL18R1 and IFNG, genes that regulate type 1 T helper (TH1) cells in patients with COVID-19. Further analysis showed upregulation of the complement pathway in TH1 cells, confirming a prior observation in lung CD4+ T cells6. CD46, the receptor for complement 3b, which has been previously show to regulate the production of IFN-γ and interleukin-10 (IL-10) by CD4+ T cells7, was also upregulated in the TH1 cells of patients with COVID-19. However, in individuals with severe COVID-19, IL10 expression was undetectable in TH1 cells, suggesting that IL-10 expression—and possibly CD46 signaling—was perturbed. Co-activation of the T cell receptor and CD46 leads to expression of IL-10 in TH1 cells.
Twenty-four transcription factors were enriched in these cells, including VDR, a member of the nuclear hormone family of transcription factors. Vitamin D has been shown to negatively regulate T cell populations such as TH17 cells8 and TH2 cells9, but the effects on TH1 cells were unexpected. Moreover, the authors5 found that the T cells also expressed CYP27B1, which encodes the 1α-hydroxylase enzyme that converts 25-hydroxy vitamin D (25(OH)D) to its active di-hydroxylated form. The importance of CD46 in regulating both VDR and CYP27B1 was confirmed by studying T cells from CD46-deficient patients.
Next, the authors showed in vitro that active di-hydroxylated vitamin D could repress IFN-γ production and induce IL-10 production in human CD4+ T cells (Fig. 1). As evidence of functional CYP27B1 expression in this ex vivo system, the authors demonstrated that inactive 25(OH)D3 could recapitulate the active vitamin D findings. Vitamin D induced known genes such as those encoding the immunoregulatory molecule CTLA4 and the enzyme CYP24A1—the latter being a biomarker of vitamin D activity10—and repressed both type 1 genes and type 17 genes, consistent with prior work with TH17 cells8. The authors did not observe the emergence of a regulatory T cell population (either type 1 regulatory (Tr1) or T regulatory (Treg) cells) in this system, but observed potent induction of STAT3 by vitamin D as well as the STAT3 activator IL-6. Blockade of the IL-6 receptor (IL-6R) or antagonism of vitamin D induced STAT3 activation and reduced IL-10 production in CD4+ TH1 cells.
Vitamin D treatment also resulted in epigenetic alterations in CD4+ T cells, including increased open chromatin (identified by H3K27ac CUT&RUN assays) in areas that were enriched for superenhancers associated with STAT3, BACH2 and IL10. JUN, VDR, STAT3 and BACH2 binding were increased in vitamin-D-treated cells across many regions of chromatin, as assayed by CUT&RUN or CUT&TAG, demonstrating that there was a profound effect of vitamin D on CD4+ T cells. Using chromatin immunoprecipitation–polymerase chain reaction (ChIP–PCR), the authors demonstrated that VDR itself binds to the STAT3 promotor, as well as the positive control CYP27A1. Using BACH2 haplo-insufficient human cells and cells from Bach2–/– mice showed that BACH2 was critical for IL6R induction by vitamin D, as well as BACH2 binding to the Il10 locus.
Returning to COVID-19, the authors noted that steroids such as dexamethasone can increase VDR expression11 and suggested that some of the effects of dexamethasone may be through this pathway. They found evidence that vitamin-D-repressed genes were enriched in patients wtih COVID-19 compared to healthy controls, with an association to TH1 genes. The authors did not find any differences in vitamin-D-enhanced genes between COVID-19 and healthy controls, suggesting that the data were more specific for the repressed dataset. It is unclear whether or not CYP24A1 was differentially regulated.
Could vitamin D be used to treat severe COVID-19? Except for some support in open-label trials, there is only limited evidence of a therapeutic benefit of vitamin D in COVID-19. One small randomized controlled trial with 25(OH)D in subjects with COVID-19 with serum 25(OH)D levels of <30 ng ml–1, showed that supplementation with oral 25(OH)D3 was associated with preserved lymphocyte counts in blood and an increased neutrophil-to-lymphocyte ratio12. There was a trend to a reduced number of days of intensive care utilization in the vitamin D arm, but this was not statistically significant. On the basis of their current results, Chauss et al.5 advocate for larger trials and perhaps higher doses of the vitamin D for treatment of COVID-19.
Clinical trials of vitamin D are difficult because most interventions use pro-hormones that need to be activated. The studies of Chauss et al.5 identify novel, but extremely complex, pathways regulated by VDR in CD4+ T cells. They also provide important tools that may enable the profound effects of vitamin D to be exploited for clinical benefit in severe COVID-19. Although healthy CD4+ T cells express CYP24B, we do not know how functional this enzyme is in the context of disease. Once active vitamin D is converted to its active form, it can bind cytoplasmic VDR, translocate to the nucleus and regulate gene expression. But this latter effect is likely to be quite different in naive CD4+ T cells versus effector cells or in tissue-resident memory cells. Thus, timing of vitamin D intervention may be critical. We currently do not know whether the exaggerated type 1 response is some individuals is an over-exuberant response or a response that was insufficient early on, leading to a too-little-too-late exuberant response.
Dosing with vitamin D would also be a challenge as the level needed to treat bone disease (>30 ng ml–1) may be insufficient for immune-modulatory effects. An important caveat is that serum levels of 25(OH)D may not reflect tissue levels of active vitamin D10. The dataset in this paper identifies genes and epigenetic markers that may serve as important biological readouts informing T-cell-intrinsic vitamin D status.